Accueil » Anesthesia products » Infusion Port
Implanted Infusion Port
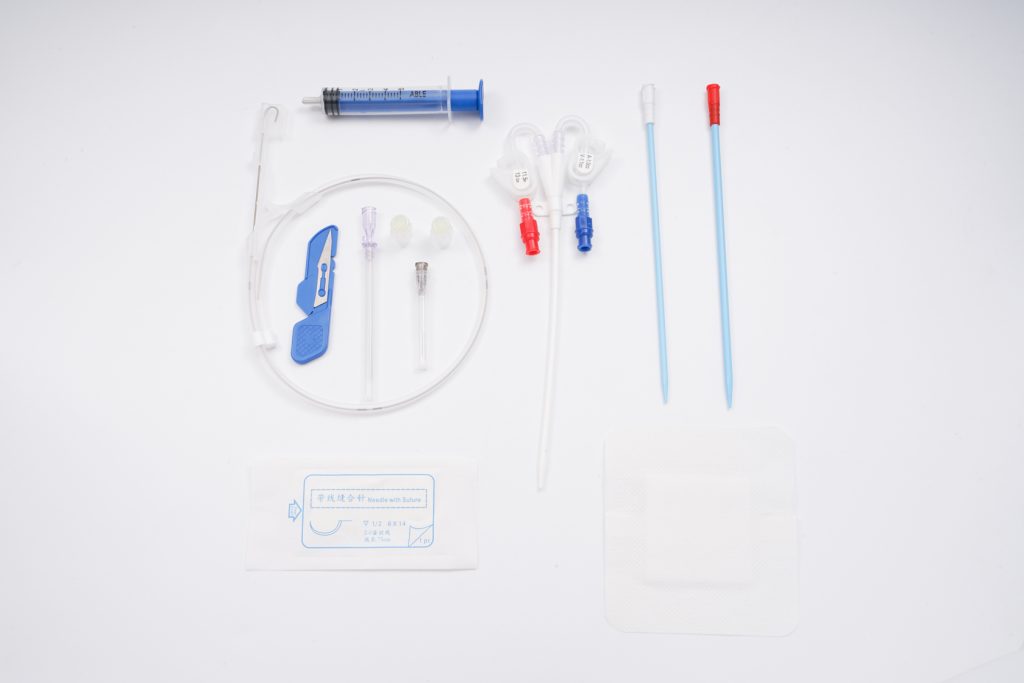
Infusion Port is a closed intravenous infusion system that is fully implanted in the body. It can be used for long-term intravenous infusion, intravenous injection of nutritional solution and blood products, infusion of various drugs, and blood sample collection in clinic.
FEATURES
Model Specificity: Features can vary between specific port models within the Baihe product line. Always refer to the official product labeling, Instructions for Use (IFU), or consult directly with Baihe Medical for the exact specifications of a particular model.
Regulations: Features and availability might differ based on regional regulatory approvals (e.g., NMPA in China, FDA in USA, CE in Europe).
Professional Use: Implantation and management require trained healthcare professionals.
- Implanted & Concealed: Under the skin, no external parts.
Components:
Port Reservoir (Body/Chamber): A small, disc-shaped chamber made of Titanium: Strong, biocompatible, and MRI-safe. Contains a self-sealing silicone septum at the top.
Catheter: A thin, flexible tube made of unique TPU material connected to the port reservoirDurable Septum: Self-sealing, withstands hundreds of punctures.
Central Access: Catheter tip in large central vein.
Long-Term: Designed for years of use.
Low Profile: Minimal visibility/bulk.
Reduced Infection Risk: Compared to external catheters.
Convenience: For frequent/long-term IV therapy and blood draws.
Needle Access: Requires a specific non-coring (Huber) needle.

High-Quality Materials
Port Body/Base: Port Reservoir (Body/Chamber): A small, disc-shaped chamber made of Titanium: Strong, biocompatible, and MRI-safe. Contains a durable, self-sealing silicone septum at the top. Titanium offers excellent biocompatibility, MRI compatibility (under specific conditions), and durability. Septum: Made from medical-grade silicone rubber. Designed for high durability and self-sealing after hundreds to thousands of needle punctures. Catheter: Primarily made from TPU, provides higher tensile strength and pressure resistance.

Power Injector Compatibility (CT Compatibility)
Designed and tested to withstand the high pressures generated during power injection for contrast-enhanced CT scans. A critical feature for oncology patients who frequently require diagnostic imaging. Specific pressure ratings (300 psi, 5 mL/sec).

Safety & Anti-Reflux Features
Incorporate anti-reflux valve technology within the catheter tip. This valve: Opens under positive pressure during infusion. Closes when infusion stops or under negative pressure (like during blood aspiration), preventing blood reflux into the catheter tip. Aims to reduce the risk of catheter-related thrombosis and occlusion, potentially decreasing maintenance requirements (e.g., less frequent heparin flushes).

MRI Compatibility
Radiopacity facilitates confirmation of catheter placement. The multi-lumen version's tips are more radiopaque, which makes it easy to confirm the placement of fluoroscopic tip.
Disposable Endotracheal Tube
1. The main raw material of the product is medical-grade polyvinyl chloride (PVC);
2. The tube body is made of thermal sensitive material, which will become soft in the airway and is not easy to damage the airway. It has moderate hardness and elasticity and is not easy to bend;
3. The bevel angle of the tip is the standard angle, with smooth fusion head transition and no burr;
4. The periphery of the Murphy hole is smooth without sharp angle, which is not easy to damage the mucosa;
5. The connection between cuff and tube body is smooth without sharp angle. The cuff is soft, long and wide, and the pressure per unit area is small to protect tracheal mucosa.
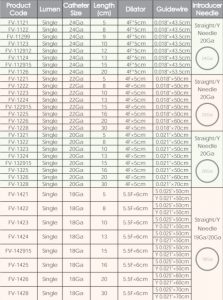
SPECIFICATIONS
8Fr, 9Fr as standard size
Single units: Kits packed in blister with Tyvek paper
Secondary packaging: Single layer corrugated paper.
Tertiary packaging: Double walled corrugated board.
DOCUMENTS